A mutation is a change in the DNA of an organism. Most mutations are harmful, but because of the redundancy of DNA (that certain codes are repeated over and over), a mutation in a single area may have only a minimal effect.
A mutation is a change in the nucleotide sequence of a short
region of a genome. Many mutations are point mutations that replace one
nucleotide with another; others involve insertion or deletion of
one or a few nucleotides. Mutations result either from errors in DNA
replication or from the damaging effects of mutagens, such as chemicals and
radiation, which react with DNA and change the structures of individual
nucleotides. All cells possess DNA-repair enzymes that attempt to
minimize the number of mutations that occur. These enzymes work
in two ways. Some are pre-replicative and search the DNA for nucleotides with
unusual structures, these being replaced before replication occurs; others are
post-replicative and check newly synthesized DNA for errors, correcting any
errors that they find. A possible definition of mutation is therefore a
deficiency in DNA repair.
A point mutation is very easy to understand. It simply means that one base is replaced with another. For example, if a part of the DNA should read TAC GGA ACT ATG but instead reads TAC GCA ATT ATG, then a point mutation has occurred in the third codon. Notice that all of the other codons remained the same. So if this part of the DNA were transcribed into mRNA which was later translated into a protein molecule, only one amino acid would be different than usual since only one codon was changed. Usually, a protein with only one amino acid different can still function normally.
By contrast to this minor effect, frame shift mutations can have much more serious consequences. A frame shift mutation occurs when a base is either added or deleted from the DNA sequence. Taking the example from before, suppose that a nucleotide with thymine was added in the second codon so that the sequence becomes TAC GGCA ACT ATG. Since the codons are triplets, this would actually be read as TAC GGC AAC TAT G... The first codon is the same, but all of the other ones are completely different, so the protein produced would also be very different from what it should be. Usually, the resulting protein will not be able to perform the function that the original one was meant to do. This is why frame shift mutations have far more severe effects than do point mutations.
Changes to individual bases______________________________________
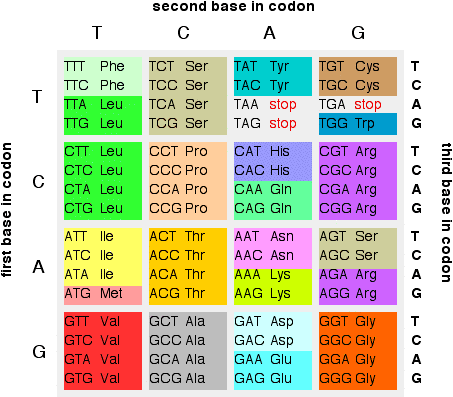
Remember that a set of three bases in a gene in DNA codes for a particular amino acid. If you have followed this sequence of pages from the beginning, you will have come across this table showing the codons in DNA:
A gene will be made up of a string of these codes rather like a
string of 3-letter words in a sentence. We'll use that as a simple
analogy. Take the sentence:
the big fox bit the dog but not the boy
Suppose one letter got changed in this by accident. Suppose, for example, the "d" in dog got replaced by a "p". The sentence would now read:
the big fox bit the pog but not the boy
Clearly this doesn't make complete sense any more. Would that matter if the same thing happened in a gene? It depends!
If you look back at the table, there are several amino acids which are coded for by more than one base combination. For example, glycine (Gly) is coded for by GGT, GGC, GGA and GGG. It doesn't matter what the last base is - you would get glycine whatever base followed the initial GG.
If you look back at the table, there are several amino acids which are coded for by more than one base combination. For example, glycine (Gly) is coded for by GGT, GGC, GGA and GGG. It doesn't matter what the last base is - you would get glycine whatever base followed the initial GG.
That means that a mutation at the end of a codon like this wouldn't
make any difference to the protein chain which would eventually form.
These are known as silent mutations.
Alternatively, of course, you could well get a code for a different amino acid or even a stop codon. If a stop codon was produced in the middle of the gene, then the protein formed would be too short, and almost certainly wouldn't function properly.
If a different amino acid was produced, how much it mattered would depend on whereabouts it was in the protein chain. If it was near the active site of an enzyme, for example, it might stop the enzyme from working entirely.
On the other hand, if it was on the outside of an enzyme, and didn't affect the way the protein chain folded, it might not matter at all.
Alternatively, of course, you could well get a code for a different amino acid or even a stop codon. If a stop codon was produced in the middle of the gene, then the protein formed would be too short, and almost certainly wouldn't function properly.
If a different amino acid was produced, how much it mattered would depend on whereabouts it was in the protein chain. If it was near the active site of an enzyme, for example, it might stop the enzyme from working entirely.
On the other hand, if it was on the outside of an enzyme, and didn't affect the way the protein chain folded, it might not matter at all.
Inserting or deleting bases_______________________________________
The situation is more dramatic if extra bases are inserted into the code, or some bases are deleted from the code. Using our example sentence from above, and keeping the three letter word structure:
If you insert a single extra base:
the big fro xbi tth edo gbu tno tth ebo y
An extra "r" is inserted in "fox". If the sentence still has to be read three letters at a time (as in DNA), everything from then on becomes completely meaningless.
If you delete a single base:
the big fxb itt hed ogb utn ott heb oy
This time the "o" in "fox" has been deleted. And again, because we have to read the letters in groups of three, the rest of the sentence becomes completely wrecked.
So does this matter? Well, of course it does! Large chunks of the protein will consist of completely wrong amino acid residues.
We've looked so far at inserting or deleting one base. What if you do it for more than one?
The effect is the same unless you add or delete multiples of three bases - without changing any other codons. If you added an extra three bases between two existing codons, then essentially you are just adding an extra word.
the big fox bit the xjy dog but not the boy
That extra word represents an extra codon in the DNA, and so an extra amino acid residue in the protein chain. Does this matter? It depends where it is in the chain (Is it important to the active site of an enzyme, for example?), and whether it affects the folding of the chain.
What if the three bases were inserted so that they broke up an existing codon? Here is the same extra "word", "xjy", dropped in the word "bit". Everything is then reshuffled into groups of three letters.
the big fox bxj yit the dog but not the boy
Deleting a whole codon again leaves most of the protein chain unchanged. Again, whether the function of the protein is affected depends on where the missing amino acid should have been and how critical it was to the way the protein folded.
Some diseases caused by mutation_________________________________
The following examples illustrate some of the changes we've looked at above and how they can result in disease.
Cystic fibrosis
Cystic fibrosis is an inherited disease which affects the lungs and digestive system. It results from mutation in a gene responsible for making a protein which is involved in the transport of ions across cell boundaries.
The effect is to produce a sticky mucus which clogs the lungs and can lead to serious infection. A similar sticky mucus also blocks the pancreas (a part of the digestive system) which provides enzymes for breaking down food. This gets in the way of the processes which convert the food into molecules which can be absorbed by the body.
There are lots of different mutations which can cause this, but we'll just have a quick look at the one which accounts for about 70% of cystic fibrosis cases.
The base sequence in the part of the gene affected ought to look like this:
The phenylalanine (Phe) in red is the amino acid which is missing from the final protein in many sufferers from cystic fibrosis. However, it isn't quite as simple as just losing the TTT codon.
Instead, the three bases lost are:
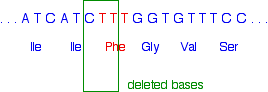
That leaves the sequence:
Notice that the amino acid sequence is identical to before but without the phenylalanine. How did that happen when we didn't actually remove the whole of the phenylalanine codon?
If you look carefully, you will see that the codon for the second isoleucine (Ile) is different from before. It so happens that isoleucine is coded for by both ATC and ATT. Once the second T (the red one) has joined the existing AT, all the rest of the base sequence is exactly what it was before.
Sickle cell anaemia
This is so called because red blood cells change their shape from the
normal flexible doughnut shape to a much more rigid sickle shape -
rather like a crescent moon.
It results from the change of a single base in a gene responsible for making one of the protein chains which makes up haemoglobin.
The affected part of the gene should read:
What it actually reads in someone suffering from sickle cell anaemia is:
The effect of this single change is to make the haemoglobin
temporarily polymerise to make fibres after it has released the oxygen
that it carries around the body. This changes the shape of the red
blood cells so that they don't flow so easily - it makes them sticky,
especially in small blood vessels. This can cause pain and lead to
organ damage.
Genetic diseases associated with defects in DNA repair systems.



















